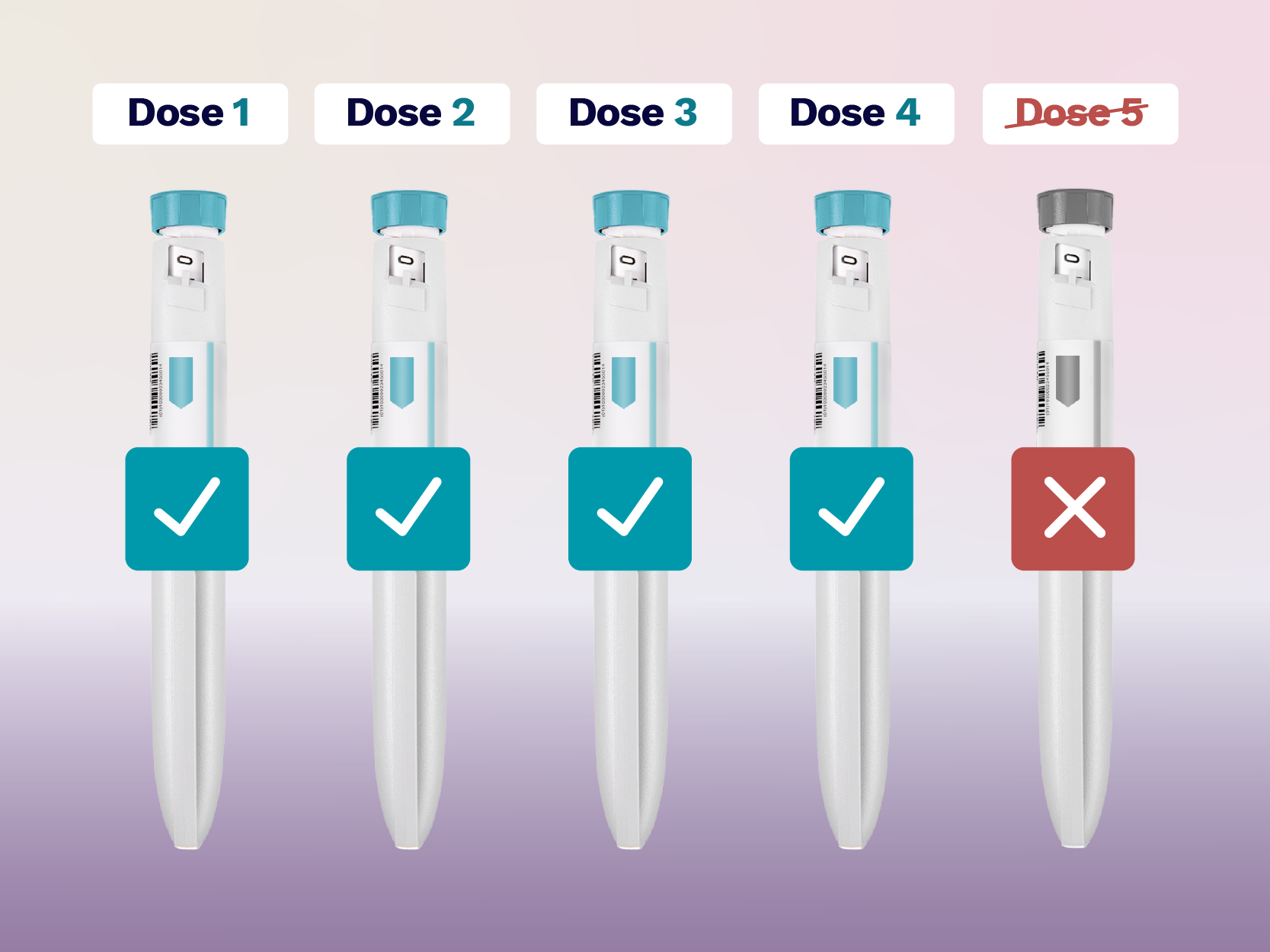
GLP-1 medications like Mounjaro and Wegovy are designed to deliver a very precise amount of medicine each week.
The ‘golden dose’ or ‘fifth dose’, refers to the small amount of medication left inside a pre-filled injection pen after the four prescribed weekly doses have been used.
This is not an actual ‘extra dose’. The leftover liquid is simply residual medication and the pen cannot safely or accurately deliver it. Pre-filled pens are engineered to deliver only the exact dose they are licensed to give, no more, no less. Anything remaining after the fourth injection is not intended to be used.
Can I inject the "golden dose"?
While it may be tempting to try to use this remaining medication, it is unsafe and not recommended.
Why is there leftover medication in the pen?
It may feel surprising that medication remains inside a single-use pen, but this is completely normal and happens with most injectable devices.
There are several reasons why this happens:
- Pen mechanics require a small overfill - manufacturers add slightly more medication than required so that the pen can reliably deliver the full prescribed dose every time.
- Needle and cartridge ‘dead space’ - some medication naturally remains in the internal components and cannot exit during injection.
- Consistency across dose strengths - each pen is calibrated so that the mechanism delivers only the stated dose, regardless of how much liquid remains inside.
The leftover medication is not a usable dose.
Why is it not recommended and unsafe to take the "fifth dose"?
- Some liquid is lost every time you prime the Mounjaro KwikPen device and there is not always the same amount of liquid left after you’ve given yourself the standard 4 Mounjaro doses.
- You do not know how much medicine you are administering with the remaining liquid also meaning you do not know how much Mounjaro you’re getting.
- If you’re using the remaining liquid “golden dose” to replace a standard Mounjaro dose, this may mean you’re getting a much smaller dose than you should be, interfering with your dosing schedule.
- It may worsen side effects before stepping up to the next Mounjaro dose.
- If you take the “golden dose” on top of your standard dose, it can also worsen side effects like feeling sick (nausea), being sick (vomiting), constipation, or diarrhoea.
What the manufacturer says about the fifth dose
Manufacturers of Mounjaro and Wegovy state that their devices are designed for just 4 fixed doses.
After you have administered the 4 doses from the pen, it should be safely disposed of in a sharps bin provided. You should not share the pen with other people, even if the pen needle is changed. A small amount of medicine may remain in the pen after all doses have been correctly given. Do not try to use any remaining medicine.
Summary - can I take the "golden dose"
Whether you use Mounjaro or Wegovy, the “golden dose” is not safe, not accurate, not approved and not a real extra dose.
For your safety and best results:
- Take only the doses the pen is designed to deliver
- Follow your prescribed weekly titration schedule
- Dispose of the pen in a sharps container after use
- Never attempt to extract leftover medication
If you have questions about dosing, side effects, or your weight loss treatment plan, Phlo Clinic can support you every step of the way.
The allure of a “free extra dose” is understandable, especially with expensive or hard-to-access medications. But when it comes to injectables like Mounjaro or Wegovy, that extra “golden dose” is a myth. The pens are carefully calibrated for a fixed number of doses, with the leftover liquid merely a safety buffer, not an extra.
Trying to squeeze out more can compromise both your health and the effectiveness of treatment. If you’re using these medications, the safest path is to follow the official dosing instructions and always dispose of pens correctly once they’ve been used.
References
Eli Lilly. How to Use Mounjaro (tirzepatide). 2025. Available from: https://mounjaro.lilly.com/how-to-use-mounjaro.
Eli Lilly. Mounjaro (tirzepatide) – Instructions for use. Available from: https://uspl.lilly.com/mounjaro/mounjaro.html#ug0
U.S. Food and Drug Administration (FDA). Mounjaro (tirzepatide) – Highlights of Prescribing Information. 2022. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/215866s034lbl.pdf#page=21
EMC. Mounjaro SPC. 2025. Available from: https://www.medicines.org.uk/emc/product/15481/pil
EMC. Wegovy SPC. 2025. Available from: https://www.medicines.org.uk/emc/product/13799/smpc